Water Transmission Rate: Teflon® Encapsulated O-rings
As an Engineer: What’s In It for You?
Water Transmission Rate; If you’re an engineer working with Teflon® FEP (fluorinated ethylene propylene) or PFA (perfluoroalkoxy), understanding how water vapor or other chemicals transmit through these fluoropolymer films is crucial for optimizing designs and ensuring product reliability. This knowledge can help you:
- Choose the optimal film thickness to control permeation and manage contamination risks.
- Predict and control moisture or chemical ingress at different pressures and temperatures.
- Improve operational safety and reduce failures in demanding or high-pressure environments.
The following narrative explains transmission properties in Teflon FEP/PFA, demonstrates how film thickness and pressure influence permeation, and shows how you can apply water transmission rate data to predict the transfer rates of other chemicals. See Engineering
1. Overview: Migration and Diffusion in Teflon® FEP and PFA
Teflon® FEP and PFA are known for exceptional chemical resistance, low surface energy, and robust barrier capabilities. However, small molecules—like water vapor—still migrate or diffuse through these polymers if a concentration or pressure gradient exists. Key factors influencing transmission include:
- Film Thickness: Thicker films slow down molecular transfer by increasing the path length.
- Temperature: Higher temperatures boost molecular mobility, accelerating diffusion.
- Pressure (Partial Pressure Difference): A larger pressure gradient increases the driving force for migration.
- Permeant Properties: Each chemical has its own diffusion coefficient and solubility in the fluoropolymer
2. Relationship of Water Transmission to Film Thickness
In barrier testing, the water vapor transmission rate (WVTR) through Teflon FEP/PFA is often measured. Observationally:
WVTR∝1thickness.\text{WVTR} \propto \frac{1}{\text{thickness}}.WVTR∝thickness1.The intuitive reason is straightforward: a thicker film presents a longer diffusion path, reducing the rate at which water molecules can migrate from one side to the other.
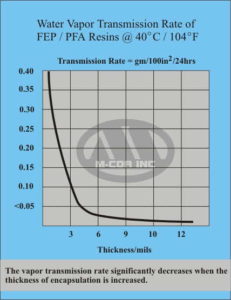
Permeation Rates
3. Using Water as a Baseline for Other Chemicals
Why Water?
- Universal Benchmark: Water vapor transmission is an industry-standard test for polymer films.
- Consistent Data: WVTR values at various thicknesses and temperatures are readily available.
- Benchmark for Comparisons: If you know how fast water transmits, you gain a reference point to gauge other chemicals’ rates.
How to Compare
If you understand water’s transmission at a given thickness and temperature, you can qualitatively predict how other substances might behave:
- Smaller, more soluble molecules might have faster transfer.
- Larger, less polar molecules tend to have slower transmission.
- Chemical polarity and interaction with Teflon influence permeation
4. The Importance of Pressure
Pressure differences (or partial pressure gradients) drive permeation just as strongly as temperature and thickness. In many applications—especially where pressurized gases or fluids are contained—pressure can be the dominant factor. Specifically:
- Higher internal pressure increases the concentration gradient across the film, accelerating the rate at which molecules diffuse.
- Lower external pressure (or vacuum) on one side can similarly increase the gradient, drawing molecules through the polymer.
- The effective force for transmission is Δp\Delta pΔp, the difference in partial pressures between the two sides of the film (for vapors) or difference in total pressure (for gases).
In short, even if the temperature remains constant, changing the pressure on one side of your system can drastically alter how fast permeation occurs.
5. Impact of Elevated Temperatures
In addition to pressure, temperature profoundly affects transmission:
- Higher temperatures typically make both the polymer chains and the permeant molecules more dynamic, reducing the energy needed for diffusion.
- Teflon FEP/PFA can soften slightly with heat, allowing molecules to migrate more rapidly.
- An Arrhenius model is often used to estimate how transmission rates rise with temperature.
6. General Formula for Transmission
A straightforward equation for permeant flux JJJ through a polymer film is:
J = P ΔpL,J \;=\; \frac{P \,\Delta p}{L},J=LPΔp,where:
- JJJ = Flux (amount of permeant passing through per unit area per unit time).
- PPP = Permeability coefficient (unique to each polymer–permeant combination).
- Δp\Delta pΔp = Partial pressure difference driving the permeation.
- LLL = Film thickness.
From this formula, we see how film thickness inversely affects the rate, and how the pressure gradient (Δp\Delta pΔp) directly drives transmission. When substituting water for another chemical, the primary change is in PPP, reflecting that different molecules have different diffusion and solubility parameters in Teflon.
If you need to consider temperature, you can factor it in via an Arrhenius-style expression for PPP:
P(T) = P0 exp (−EaRT),P(T) \;=\; P_0 \,\exp\!\Bigl(-\frac{E_a}{RT}\Bigr),P(T)=P0exp(−RTEa),where EaE_aEa is the activation energy for permeation, RRR is the gas constant, and TTT is the absolute temperature (K).
7. Practical Steps and “What’s in it for Me” Takeaways
- Gather Water Transmission Data
- Measure or look up the WVTR of your Teflon FEP/PFA film at the operating thickness, pressure, and temperature ranges.
- Establish a Baseline
- Use that measured data to identify an effective permeability for water.
- Understand how thickness, temperature, and pressure differentials affect the measured rate.
- Compare with Other Chemicals
- Adapt the formula using different permeability coefficients (or look up known data).
- Consider differences in vapor pressure or partial pressures for each chemical.
- Account for Pressure-Driven Effects
- If your process involves elevated or reduced pressure, calculate or test how that changes the gradient (Δp\Delta pΔp).
- Recognize that even a small increase in Δp\Delta pΔp can significantly raise transmission.
- Optimize Your Design
- Increase film thickness if you need to reduce permeation.
- Select the appropriate grade of Teflon FEP/PFA if certain chemicals or pressure levels demand it.
- Validate with Real Testing
- Confirm actual transmission rates under real-world conditions (temperature, pressure, chemical environment).
- Adjust or refine your models based on these test results.
8. Conclusion
By using water transmission rates as a straightforward reference point, you can develop practical insights into how Teflon FEP/PFA films behave under varying pressures and temperatures. This answers the core question, “What’s in it for me?” by equipping you to:
- Select the right film thickness to control diffusion and migration.
- Predict how pressure and temperature changes alter transmission rates.
- Design safer, more reliable systems that minimize unwanted penetration of moisture or chemicals.
Ultimately, leveraging this information allows for better-informed engineering decisions, reduced risk, and enhanced performance in applications that rely on Teflon’s robust barrier properties.
